Haloform reaction
Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom (methyl ketones) and secondary methyl carbinols with halogen are oxidised by sodium hypohalite to sodium salts of corresponding carboxylic acids having one carbon atom less than that of carbonyl compound. The methyl group is converted to haloform. This oxidation does not affect a carbon-carbon double bond, if present in the molecule.
Haloform reaction is specific for methyl ketones and alcohols oxidisable to methyl ketones .Thus haloform reaction constitutes a classical test for methyl ketones and methyl carbinols.
The reaction is carried out by dissolving the compound in dioxane and first adding dilute sodium hydroxide and then a slight excess of iodine in potassium iodide solution.this is the followed by slight warming and finally adding water. If the compound contains the acetyl group , iodoform is precipitated out. when a ketone is treated with sodium hypoiodite solution , iodoform and sodium salt of carboxylic acid is formed.
Mechanism of Haloform reaction
Haloform reaction Examples
Some Other Important Reactions
Aldol condensation
Benzoin condensation
Birch Reduction
Cannizzaro Reaction
Clemmensen Reduction
Diels Alder Reaction
Etard Reactions
Williamson Ether Synthesis
Wurtz reaction


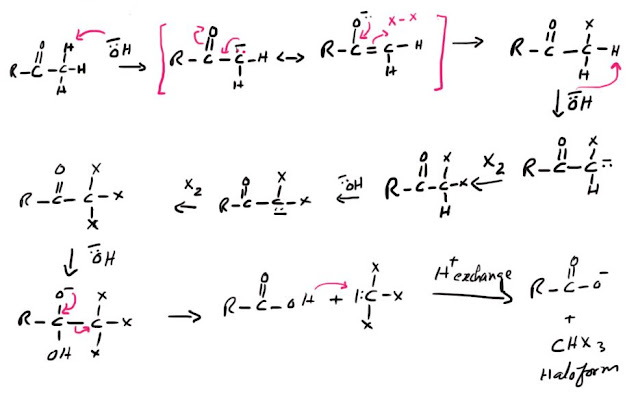
Nice blog, i really appreciate.
ReplyDeletePlease i wish to inquire about this oxidizing agent chemical called
Caluanie Heavy water, i bought from this comppany pharmawill373@gmail.com.
I wish to get some help with chemical thanks.