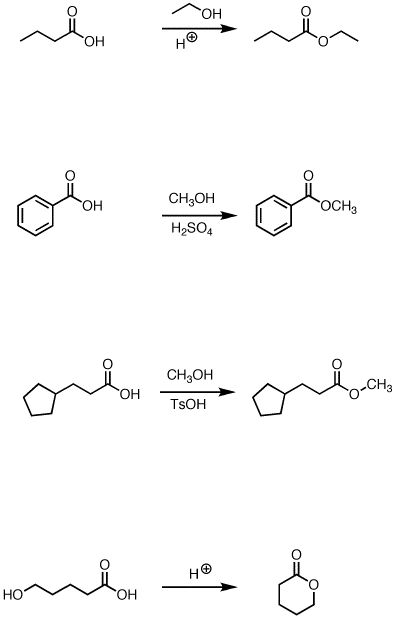
Fischer Esterification
Conversion of carboxylic acids to esters using acid and alcohols
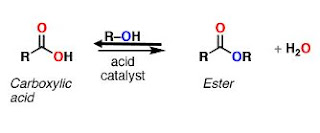 |
| Fischer Esterification |
This is a reversible reaction. The alcohol is generally used as solvent so is present in large excess.
some other examples are
The byproduct of each of these reactions is water.
The fourth example is an intramolecular reaction that forms a cyclic ester.
Cyclic esters are also called Lactones.
The reaction is useful in
the synthesis of esters, which are commonly used as fragrances, flavors, and
solvents. The reaction can also be used to convert carboxylic acids into their
corresponding esters, which can be more volatile
and easier to handle than the
parent acid.
However, Fischer
esterification has some limitations, such as the fact that the reaction is
reversible and the equilibrium can be shifted towards the starting materials.
Additionally, the reaction is sensitive to the presence of water,
which can
hydrolyze the ester and shift the equilibrium back towards the
starting
materials.