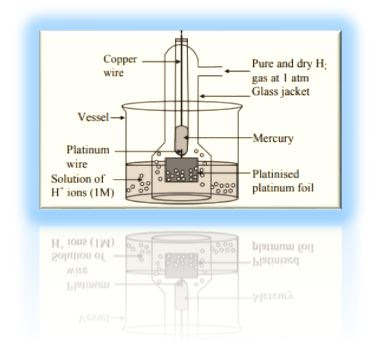
Standard Hydrogen Electrode
Reference Electrode
It is not possible to measure the absolute value of the single electrode potential directly. Only the difference in potential between two electrodes can be measured experimentally. It is, therefore, necessary to couple the electrode with another electrode whose potential is known. This electrode is termed as reference electrode. The commonly used reference electrode is Standard Hydrogen Electrode (SHE) or also called as Normal Hydrogen Electrode (NHE).
Standard Hydrogen Electrode, (SHE)
Ø It consists of a small platinum strip
coated with platinum black as to adsorb hydrogen gas.
Ø The platinum strip is placed in an acid solution which has H+ ion
concentration 1 M.
Ø A platinum wire is welded to the
platinum strip and sealed in a glass tube as to make contact with the outer
circuit through mercury.
Ø Pure hydrogen gas at one atmospheric
pressure is constantly bubbled through the solution.
Ø The hydrogen electrode thus obtained
forms one of two half-cells of a voltaic cell. When this half-cell is connected
with any other half-cell, a voltaic cell is constituted. The hydrogen electrode
can act as cathode or anode with respect to other electrode.
Ø When it acts as anode the cell
reaction that occurs at this electrode is
H2
2H+ + 2e-
Ø When it acts as cathode the cell reaction that
occurs at this electrode is
2H+ + 2e → H2
The
temperature of the cell is maintained at 250C. By
international agreement the standard hydrogen electrode is arbitrarily assigned
a potential of exactly ± 0.000 Volt.
Determination of Standard Electrode Potential of Zn/Zn2+ Electrode
An
Electrochemical Cell Is Setup in which a zinc rod is dipped in 1 M zinc Sulphate
solution. This half-cell is combined with a standard hydrogen electrode through
a salt bridge. The deflection of the voltmeter indicates that current is
flowing from hydrogen electrode to metal electrode or the electrons are moving
from zinc rod to hydrogen electrode. Hence the zinc electrode acts as an anode and the hydrogen electrode acts as cathode .in this case the reading (EMF) by the
voltmeter is 0.76 V. The cell can be represented as
Zn + 2H+ → Zn2+ +H2
Calculations
The EMF of the cell is 0.76 volt
ECell = EoAnode + EoCathode
0.76 = EoAnode + 0 or EoAnode = +0.76 V
Hence
oxidation potential of zinc is 0.76 V and reduction potential of zinc will be –
0.76 V
Determination of Standard Electrode Potential of Cu2+/Cu, Electrode:
An
Electrochemical Cell Is Setup in which a copper rod is dipped in 1 M copper Sulphate
solution. This half-cell is combined with a standard hydrogen electrode through
a salt bridge. The deflection of the voltmeter indicates that current is
flowing from copper electrode to hydrogen electrode or the electrons are moving
from hydrogen rod to copper electrode. Hence the copper electrode acts as an
cathode and the hydrogen electrode acts as anode. In
this case the reading (EMF) by the voltmeter is 0.34 V. The cell can be
represented as
Cu2+ + H2 → 2H+ + Cu
Calculations
The
EMF of the cell is 0.34 volt
E Cell
= Eo Anode + Eo Cathode
0.34
= 0.0 + Eo Cathode or Eo cathode= +0.34 V
Hence reduction
potential of copper is 0.34 V and oxidation potential of copper will be – 0.34V.