BOYLE’S LAW
According to this law, for
a given sample of a gas, the pressure is inversely proportional to its volume
at constant Temperature. That is, if we increase the volume then the pressure
decreases and vice-versa. This is obvious as on increasing volume the contact
area increases and that’s why pressure decreases. The force remains the same as
the temperature is constant.(BOYLE’S LAW).
P ά 1/V
PV = constant
P1V1
= P2V2 = P3V3
If the
pressure is doubled, the volume is halved. If the pressure is halved, the volume
is doubled.
The study of graphs plays a vital role in
understand these laws.
The P-V graph is called “rectangular hyperbola” as
it represents a standard curve which follows the equation xy = constant.
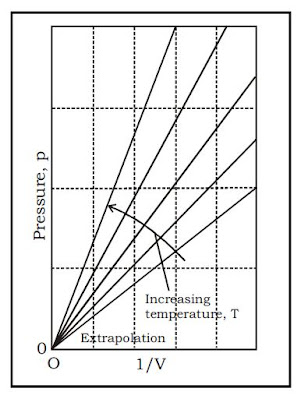
Straight lines are obtained
when the pressure is plotted against 1/V at constant temperature.
The P-V graph is called “rectangular hyperbola” as it represents a standard curve
which follows the equation xy = constant. As they are drawn at constant T, these curves are also called “Isotherms”.
Experiments of Boyle, in a quantitative manner prove that gases are highly
compressible because when a given mass of a gas is compressed, the same number of molecules occupy a smaller space. This means that gases become denser at high pressure.
A relationship can be obtained between density and pressure of a gas by using Boyle’s law . By definition, density ‘d’ is related to the mass ‘m’ and the volume ‘V’ by the relation d = m / V. If we put value of V in this equation from Boyle’s law equation, we obtain the relationship.
d ={m/k}P =K' P
This shows that at a constant temperature, pressure is directly proportional to the density of a fixed mass of the gas.
Example : A balloon is filled with hydrogen at room temperature. It will burst if pressure exceeds 0.2 bar. If at 1 bar pressure the gas occupies 2.27 L volume, upto what volume can the balloon be expanded ?
Solution
According to Boyle’s Law P1V1 = P2V2
If P1 is 1 bar, V1 will be 2.27 L
If P2 = 0.2 bar, then
V2 = 1 bar x 2.27 x 1/0.2 = 11.35L
Since balloon bursts at 0.2 bar pressure, the volume of balloon should be less than 11.35 L.
Example:
A 1.00 L sample of gas at 760 torr is compressed
to 0.800 L at constant temperature. Calculate the final pressure of the gas.
Solution. P1V1
= P2V2
(760) (1) = P2 (0.8)
P2 = 950 torr
Applications of Boyle's Law
Tags:
NEET