Carbanion
A carbanion is a species containing unshared pair on central carbon atom and
three groups attached to the Carbon. As there are three groups on carbon and
unshared pair on carbon, carboanion is negatively charged . Carboanion has
eight electrons in its outermost orbital, sp3 hybridized, pyramidal shaped and
act as nucleophile.
Carboanion has eight electrons in its outermost orbital, sp3 hybridized,
pyramidal shape and act as nucleophile . Formally carboanion is a
conjugated base of carbon acid.
Stability of Carboanion
Stability of carboanion depends on many factors.
1. Electronegativity of Carboanionic carbon .When elctronegativity of
carboanionic carbon increases, stability of carboanion increases.
Electronegativity of carboanion depends on % s-character of carboanionic
carbon. % s character depends on hybridization of carbon and increase in
following order;
Therefore, stability of carboanion is high when carboanionic carbon is sp hybridized.
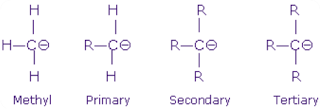
2. Inductive effect of substituents attached to the carboanionic carbon
There are two inductive effects operate in organic compounds , +I and –I.
When groups with +I effect is attached to the carboanionic carbon, stability
of carboanion decreases. Therefore, tertiary carboanion is the least
stable and methyl carboanion is the highest stable as shown below.
When group or atom with – I effect is attached to the carboanionic carbon,
the stability of caroanion increases. Electronegative atoms or groups with
electronegative atoms are connected to carboanionic carbon, stability
of carboanion increases. Therefore, following carboanions are very stable.
3. Delocalization or Resonance.
When carboanion is in conjugation with double bond those carboanins
are stable due to resonance as shown in following figure.
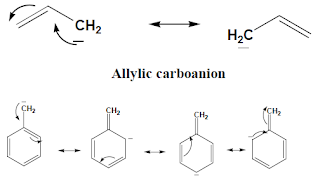 |
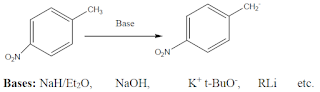
| Benzylic Carboanion |
4. Stabilization by aromaticity
Carboanions are stabilized by aromaticity. Following carboanion is stable
as it is aromatic. When carboanion is conjugated with carbonyl group
(enolate ion) or nitrile group, these carboanions are stable due to resonance
and –I effect of electronegative atoms.
Generation of Carboanions
There are many of ways of generating carboanions
1. From Grignard reagent(RMgX). Grignard reagent is good source for carboanion.
2. Metal Halogen interchange reaction
Organo metallics are good sources for carboanions. Metal-halogens exchange reactions
are frequently used to prepare vinyl and aryllithium.
Tags:
Organic





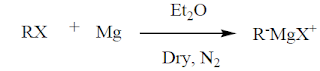


This comment has been removed by the author.
ReplyDelete