Nucleophilic Aromatic Substitution Reaction
We have learnt many examples of electrophilic aromatic
substitution. What about nucleophilic aromatic substitution? Under what conditions
it may be possible for a nucleophile to add to an aromatic ring to give a
cyclohexadienyl anion that could subsequently rearomatize by expelling some
negatively charged leaving group (LG)?
Nucleophilic substitution is not a typical reaction of aromatic compounds as the presence of the pie-electron system in their sigma-bond framework repels the unshared electron pair of the nucleophile.However, appropriately substituted aromatic compounds do undergo nucleophilic substitution.
The reactions that are successful at an aromatic substrate are largely of four kinds:
The reactions that are successful at an aromatic substrate are largely of four kinds:
1. Reactions activated by electron withdrawing
groups ortho and para to the leaving group
2. Reactions catalyzed by very strong bases
3. Reactions initiated by electron donors
4. Reactions in which the nitrogen of a diazonium
salt is replaced by a nucleophile.
Nucleophilic Aromatic Substitution Mechanism
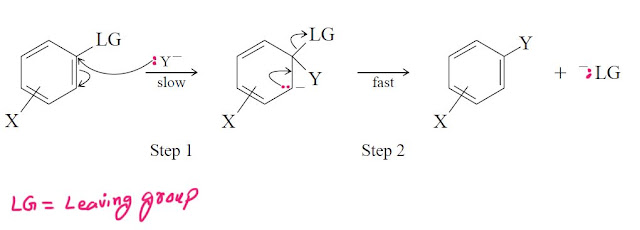
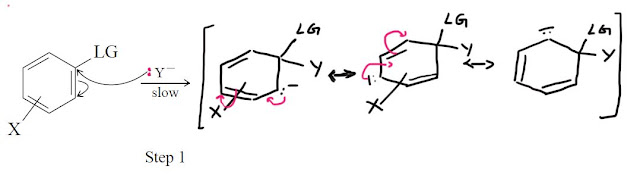
The SNAr mechanism. by far the most important mechanism for aromatic substitution consists of two steps:
Like other SN2 reactions these substitution reactions follow second order kinetics.A variety of evidence suggests that the reaction goes through an addition-elemination process involving the attack of the nucleophile in the first step.In the second step of the reaction this intermediate loses the anion thereby regenerating the aromatic character.
Nucleophilic aromatic substitution practice problems
Nucleophilic aromatic substitution leaving group
The common leaving groups in aliphatic substitution are also the common leaving groups in aromatic nucleophilic substitution, however the groups like NO2 , OR , OAr, SO2R,SR which are not generally lost in aliphatic systems, are leaving groups when attached to aromatic rings.
The approximate order of leaving-group ability is F> NO2 > OTs> SOPh > Cl,Br,I> N3> NR3. However this depends greatly on the nature of the nucleophile.
The leaving group order is quite different from that for the SN1or Sn2 mechanism.the most likely explanation is that the first step of the SNAr mechanism is usually rate determining and this step is promoted by the groups with strong -I effect.
Fluoro is the the poorest leaving group of the halogens when the second step of the SNAr mechanism is rate determining or when the benzyne mechanism is operating.