Oxidation of alcohols
Alcohol can be oxidized to aldehydes and ketones or carboxylic
acids. The nature of oxidation products depends on whether the alcohol is
primary secondary or tertiary.
A primary alcohol is it oxidized to an aldehyde and then when acid
both containing the same number of carbon atoms as the original alcohol.
The oxidation of aldehyde to carboxylic acid in aqueous solution
is easier than oxidation of primary alcohol to aldehydes; that's it is
difficult to stop oxidation at the aldehyde state. Therefore most Laboratory
preparations we must rely on special conditions to prepare aldehydes from
alcohols. a variety of reagent is
available. An excellent reagent for this purpose is pyridinium chlorochromate (abbreviated
PCC), the compound formed when CrO3 is dissolve in hydrochloric acid and then
treated with pyridine.
PCC, when dissolved in CH2Cl2 will oxidize a primary alcohol to an
aldehyde and stop at that stage:
Pyridinium chlorochromate also does not attack double bonds.
Oxidation of primary alcohols to carboxylic acids.
Primary alcohols can be oxidized to carboxylic acids by potassium
permanganate or potassium dichromate.
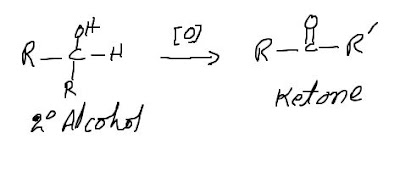
Oxidation of secondary alcohols to ketones:
Secondary alcohols can be oxidized to ketones. The reaction
usually stops at the Ketone stage because further oxidation requires the
breaking of a carbon-carbon Bond.
Jones Reagent: The most commonly used reagent is chromic acid (H2CrO4),
chromic acid is usually prepared by adding CrO3 to aqueous sulfuric acid.