Acids and bases
Bronsted theory:
According to this theory an acid is defined as a Proton Donor and a base as a Proton acceptor. An acid-base reaction is simply the transfer of proton from an acid to a base. When the acid gives of a Proton, the species remaining still retains the electron pair to which the proton was formally attached. Thus the new species can we acquire a Proton and is therefore base. It is referred to as the conjugate base of the acid. All acids have a conjugate base, and all bases have a conjugate acid. All acid-base reactions can be shown as given below;
A-H + B: ---> A: + B-H
Acid Base Conjugate base Conjugate acid
Acid strength may be defined as the tendency to give up a Proton and a base strength as a tendency to accept a Proton.
The molecule or ion that forms when an acid loses its proton is called the conjugate base of that acid. The Chloride ion therefore is the conjugate base of hydrochloric acid. The molecule or ion that forms when a base accepts a Proton is called the conjugate acid of that base. The hydronium ion therefore is the conjugate acid of water.
H2O + H-Cl ----> H3O+ + Cl-
Proton acceptor Proton Donor
Among the factors that may influence the acidity of an organic compound are
- The strength of H-A Bond
- The electronegativity of A
- Factors stabilizing A-
- The nature of solvent
The strength of an acid H-A in water may be determined by considering the equilibrium
H2O + H-A <----> H3O+ + A-
H2O + H-A <----> H3O+ + A-
Then the equilibrium constant in water is given by
Ka = [H3O+ ] [A-] / [H-A ]
A large value of ka means the acid is strong and small value of Ka means acid is a weak acid.
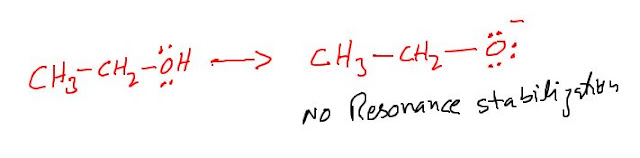
The acidity of carboxylic acids
Both carboxylic acid and alcohol or weak acids. however Carboxylic acids are much more acidic than corresponding alcohols. the greater acidity of the Carboxylic acid is primarily due to resonance stabilization of the carboxylate ion.The greatest stabilization of anion of a carboxylic acid lowers the free energy of the anion and thereby decreases the positive free energy change required for the ionization.
Any factor that makes the free energy change for the ionization of an acid less positive makes the acid stronger