Simmons Smith reaction mechanism
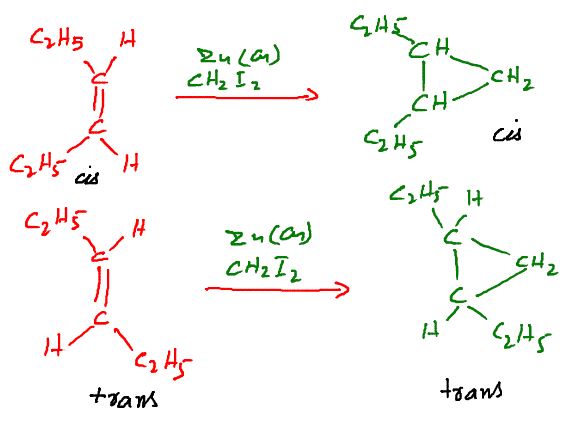
The reaction involves the treatment of an olefinic compound with diiodomethane (CH2I2) and Zn-Cu couple. the attacking species is an organozinc intermediate , a carbene like species called a carbenoid. Generally substituted alkenes react somewhat faster than unsubstituted alkenes.
Thus , 1-methylcuclohexene reacts faster than cyclohexene. The reaction is stereospecific and occurs by the cis addition of methylene to the less hindered side of the double bond.
Simmons Smith Reaction mechanism is not clear, but metal
carbenoid is likely to be involved.



Thank you Cinetux sharing the technical graphics it will be very helpful for students and other regarding machenism.
ReplyDelete