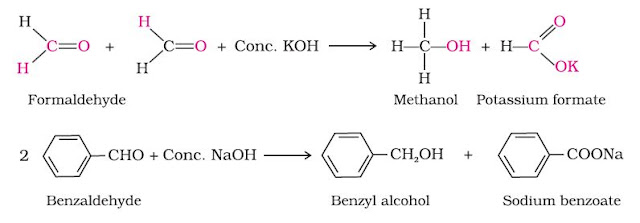
Cannizzaro reaction:
Aldehydes which do not have an α-hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on treatment with concentrated alkali. In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
Cannizzaro Reaction Mechanism:
The mechanism most likely involves the formation of reducing anion (1) by the interaction of the aldehyde with hydroxide ion. This ianion may transfer a hydride ion intermolecularly to a carbonyl carbon of another molecule , forming the carboxylic acid and alkoxide ion (2). the shift of proton from acid to (2) gives the final product.
A mixture of two aldehydes having no α-hydrogen undergo crossed cannizzaro reaction to yield all possible products. However , if one or the aldehyde is formaldehyde , which is more reactive than other aldehydes towards nucleophiles and rapidly give a high concentration of the donor anion, the products are sodium formate resulting from the oxidation of formaldehyde exclusively and the alcohol corresponding to the other aldehyde.Thus, crossed cannizzaro reaction between benzaldehyde and formaldehyde yields benylalcohol and sodium formate.
Some other important reaction
Aldol condensation
Some other important reaction
Aldol condensation