Organic free radicals are important reactive intermediates and have been used in synthesis of many important compounds.The term radicals applies to any species which contain an unpaired electrons. Radicals are usually neutral intermediates, paramagnetics and can be observed by ESR.
Structure of Radical
Radicals can be C-centered or heteroatom centered radicals. Radicals can be either plannar geometry or pyramidal geometry depending on hybridization of central atom.
1. Plannar geometry with sp2 hybridized central atom
2. Pyramidal geometry with sp3 hybridized central atom
Stability of Radical
Radicals are very reactive species but they exhibit some stability. Therefore, radicals have been
used as very effective reactive intermediates for synthesis of many compounds. The structural
factor is very important with the stability.
There are various types of Organic radicals;
a) Alkyl radical
b) Allyl radicals
c) Benzyllic radicals
d) Heteroatom substituted radicals etc.
Alkyl radical
There are primary, secondary and tertiary alkyl radicals. The stability of alkyl radicals are in the same order of alkyl carbocations. Thatmean, tertiary radical is the most stable.
The stability can be explained using the concept “Hyperconjugation” as in the case of carbocations. Tertiary radical possess more hyperconjugation than secondary radical and very low in primary radical.
Allyl radicals
Allyl radicals are stable as allyl carbocations by resonance.
Benzyl Radical
Benzyl radicals are most stable due to resonance stabilization. The first radical identified was triphenyl alkyl radical, which is a benzylic radical. This radical is more stable due to steric effect and by resonance.
 |
| Stabilization of triphenyl methyl radical by resonance |
Generation of Radicals
Radicals are generated by homolysis of bonds. Normally radical reactions are chain reaction
process. The initial radical should be generated and then reactions proceed through a chain
process.There are mainly two ways of generating of initialradicals;
- Thermal generation
- Photochemical generation
Thermal generation
On heating some molecules undergo homolysis. There are two types of compounds which can undergo homolysis on heating,
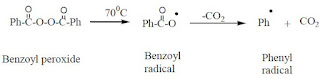
a). Compounds contain very weak bonds such as O-O, Cl-Cl, Br-Br etc.
Benzoyl radical formed can undergo decarboxylation to produce phenyl radical.
b). Compounds which on fragmentation form very stable products.
ExampleL Azo compounds produce stable molecule, N2 and radicals.
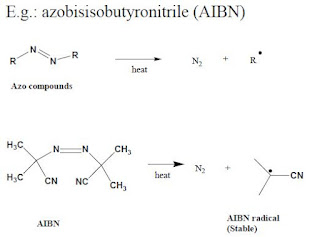 |
| Generating of radical by themerl decomposition |
Photochemical generation
Some compounds can produce radicals when they are irradiated. This can be done for the compounds which are unstable to thermal conditions. There are two conditions that must be met in order to break on irradiation.
1. The energy of light should be greater than bond energy
2. Electronic excitation of the molecules
This method is suitable for, halogens, peroxides, alkyl nitriles hypochlorides
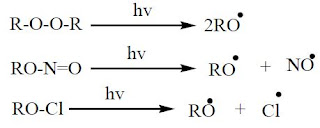 |
Photochemical generation |



Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, biochemistry, and many other chemical processes. In your blog article, you provide a great explanation of this topic. A knowledgeable expert can provide all the information on chemistry-related matters and a qualified chemistry tutor can thoroughly explain all the topics to you.
ReplyDelete