What are Carbocations?
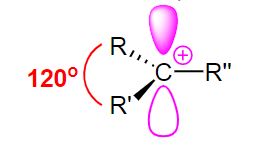
Carbocation or Carbonium ions are species with positive charge on carbon atom.
In
carbocation, carbon is trivalent and sp2 hybridized.Therefore, it has a planar geometry.
As carbon in carbocation has six electrons in its outmost orbital, it is an electron deficient
species. Therefore, carbocation acts as elctrophile.
Types of Carbocations
There are most common types of carbocations
a. Alkyl carbocations
b. Allyl Carbocation
c. Benzyl Carbocation
Alkyl carbocations
Alkyl carbocation contains alkyl groups attached to the +ve carbon atom. According to the
number of alkyl groups present, alkyl carbocations are three types;
a. Primay carbocation
b. Secondary carbocation
c. Tertiary carbocation
Their structures are shown in Figure.
Stabilty of Alkyl carbocations
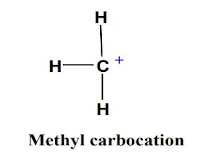
Alkyl carbocations are stable except methyl carbocation. The stabilization is due to the two
main effects, those are ;
1. Inductive effect ( I-effect)
2. Hyperconjugation
Inductive effect ( I-effect)
Carbocations are stabilized by positive Inductive effect ( +I-effect). This is the electrons
pushing effect by alkyl groups towards the +ve centre. When attached alkyl groups are
increases, stability increases due to increase of +I effect. As methyl carbocation has no any
alkyl group attached, its stability is very low.
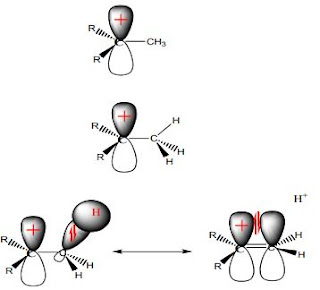
Hyperconjugation
Hyperconjugation is the sharing of sigma electrons with orbital containing positive charge or
radical. This is a hypothetical π (pi) bond between sigma orbital (from H of C-H bond) and porbital of carbocation
When number of methyl groups attached to the carbocation increases, number of
hyperconjugations increase, hence stability increases. Therefore, tertiary carbocation is the
most stable due to more hyperconjugation and higher +I effect.
Allylic Carbocation
Allylic carbon is the carbon just next to the double -bonded carbon. The cation at the allylic
carbon is named as allylic carbocation
 |
| Allylic carbocation |
Stabilty of allylic carbocation
Allylic carbocation is stable. The stabilty arises due to the delocalization of +ve charge with pi-orbital next to allylic centre. This is called resonance stabilization.
 |
| Stabilization of Allylic carbocation by resonance |
Benzylic carbocation
The carbon directly attached to the benzene carbon is called as benzylic carbon. The cation on
the benzylic carbon is called as benzylic cation
Stability of Benzylic Carbocation
Benzylic carbocation is stable and stabilized by delocalization of +ve charge with pi-orbitals in
benzene ring. This is resonance stabilization.
Generating of Carbocations :
There are different ways of generating cabocations using different substrates.
1. Solvolysis of alkyl halides:
Solvent used for the solvolysis must be polar solvents such as water or alcohol. The solvent play dual role in solvolysis reaction;
a. Act as solvent, consequently breaking of C-X bond due to polarization
b. Act as nucleophile to attack on carbocation formed
2. Using Diazonium salts
Alkyl Diazonium ions are unstable and decomposed readily to give carbocations.
3. Protonation of alcohols
Alcohols are easily protonated using dilute acids and carbocations are formed after
loosing water molecule.
4. From alkyl halides when the presence of Lewis acid catalysts , eg: AlCl3
5. From acyl chloride when the presence of Lewis acid catalysts
Rearrangement of Carbocations:
Sometime, unexpected products are formed when the reactions proceed through carbocation intermediates. This is due to the rearrangement of first formed carbocation into most stable carbocation and undergoes the second reaction to form the product. Rearrangement is most common in carbocations.
Example-1 : Pinacole-Pinacolone Rearrnagement
Tags:
Organic








nice
ReplyDelete