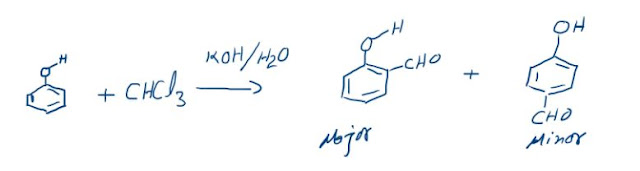
Reimer-Tiemann Reaction
Phenols on reaction with chloroform in the presence of sodium hydroxide (or
potassium hydroxide) solution give hydroxy aldehydes. The formyl group is
directed to ortho-position unless one of the ortho-positions or both are
occupied, in that case the attack is para. This formylation reaction is known
as Reimer-Tiemann reaction.
Reimer-Tiemann Reaction Mechanism
It involves the electrophilic substitution reaction and the electrophile in
this reaction is dichlorocarbene which is produced by the reaction of
chloroform with alkali. The attack of phenoxide anion on dichlorocarbene gives
benzylidene dichloride, which is hydrolyzed to the corresponding hydroxy-aldehyde.
The steps involved are shown below.
Mechanism: