ISOMERS
Isomers are different compounds
that have the same molecular formula.
Structural isomers:
Structural
isomers have the same molecular formula but different connectivity, meaning
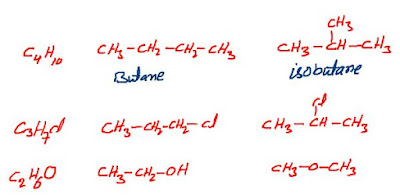
that their atoms are connected in a different order. Examples of the structural
isomers for the following;
Stereoisomers
Stereoisomers are
not constitutional isomers. Stereoisomers have the atoms connected in the same
sequence, but they differ in the arrangement of the atoms in space. Each
stereoisomer has a unique configuration (either R or S) that can only be
converted the different configuration by chemical means that is breaking and
making of bonds. Stereoisomers are further categorised into (a) conformational
isomers (b) configurational isomers
Configurational isomers of further
divided into two categories enantiomers and diastereomers;
ENANTIOMERS : Enantiomers are stereoisomers whose molecules are non-superimposible mirror images of each other.
DIASTEREOMERS : Diastereomers are stereoisomers whose molecules are not Mirrror Images of each other
ENANTIOMERS : Enantiomers are stereoisomers whose molecules are non-superimposible mirror images of each other.
DIASTEREOMERS : Diastereomers are stereoisomers whose molecules are not Mirrror Images of each other